
SCIENCE
Synaptogenix has targeted Bryostatin-1 due to its high potential and multi-modal efficacy: through protein kinase C (PKCϵ) activation, Bryostatin-1 stimulates synaptic growth factors, amyloid-β degrading enzymes, as well as prevents Tau transformation into neurofibrillary tangles.
Studies have demonstrated that PKCϵ plays a pivotal role in learning and memory. Bryostatin, a small molecule, penetrates the blood-brain barrier and activates PKCϵ, resulting in improved synaptic function, new synapse formation, repair of damaged synapses, and prevention of neuronal death. Synaptogenix’s preclinical work demonstrates improved memory and learning in multiple Alzheimer’s mouse models and several other animal models.
ALZHEIMER’S DISEASE
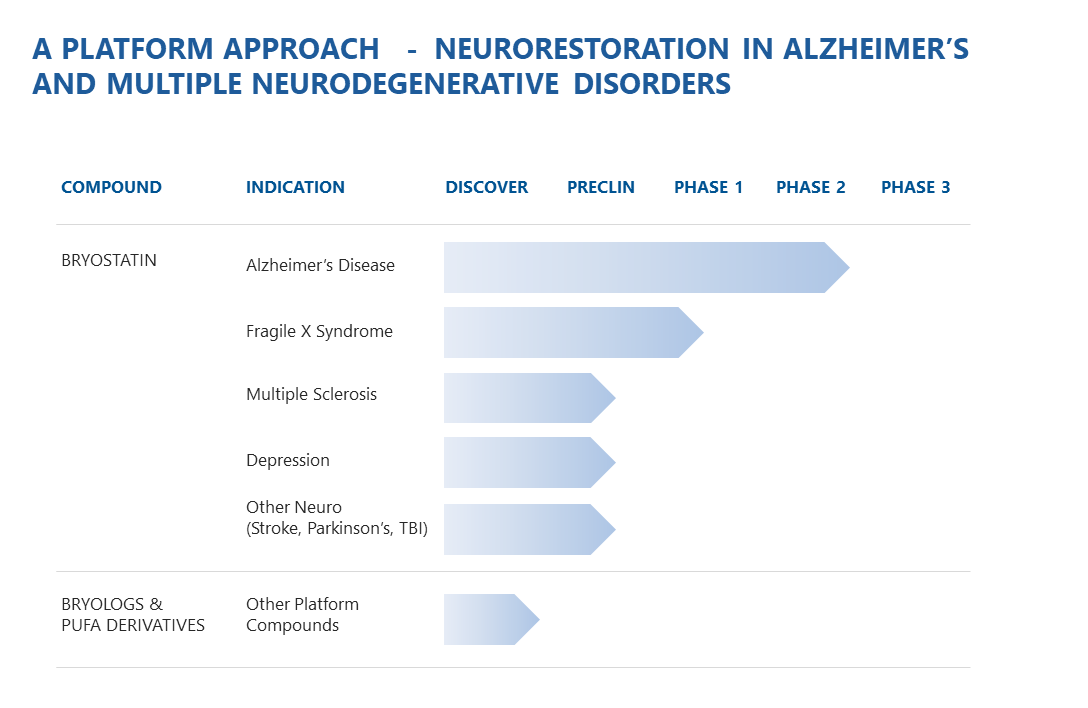
Alzheimer’s disease is the most common form of dementia, accounting for 60%-80% of all cases. Synaptogenix has conducted the first placebo-controlled, Phase 2 trial of a protein kinase C epsilon (PKCɛ) activator for the treatment of Alzheimer’s, which in preclinical studies induced the growth of new synapses and prevented neuronal death. Recent clinical trial data showed sustained increase in Severe Impairment Battery measures and improvement in cognition, suggesting the drug may treat the progression of AD, rather than just symptoms. A confirmatory trial will commence in 2018.
FRAGILE X SYNDROME
Fragile X syndrome is a genetic disorder that causes developmental delays, intellectual and learning disabilities, anxiety, and autism spectrum disorders. FXS affects 135,000 in the U.S. alone. Bryostatin-1 for Fragile X has been designated as an orphan drug, and in animal models, was shown to restore synaptic networks, rectify memory deficits, and reverse biochemical abnormalities. Synaptogenix preclinical data, in a collaboration with the FRAXA Research Foundation, showed that Bryostatin-1 significantly improves autism spectrum abnormalities. Synaptogenix plans to initiate clinical testing of FXS patients in 2018.
NIEMANN PICK TYPE C
Niemann Pick Type C is both a debilitating and lethal disease, affecting about 35,000 children per year. Synaptogenix is working with world renowned experts at the Icahn School of Medicine, Mt. Sinai to develop its lead compound, Bryostatin-1, as a potential treatment for the excessive accumulation of cholesterol and other lipids in the viscera and brain resulting in cell death and to neuologic symptoms, including difficulty in swallowing and speaking, loss of coordination, seizures, and progressive dementia. There is no FDA approved treatment for Niemann-Pick Type C.
PIPELINE
Synaptogenix is building a sustainable pipeline of treatments in major areas of unmet need.
TEAM
Synaptogenix is led by an experienced team with deep expertise in neurodegenerative disorders and successful track records in both drug discovery and development.
Alan Tuchman, M.D.
Chief Executive Officer & CMO
View ›Daniel Alkon, M.D.
President & Chief Science Officer
View ›Joshua Silverman
Chairman
View ›William Singer
Vice Chairman
View ›Jonathan Schechter.
Director
View ›Bruce Bernstein
Director
View ›George Perry, Ph.D.
Director
View ›Martin R. Farlow
(Chairman) MS
View ›Paul Coleman, PhD
Daniel F. Hanley Jr. MD
View ›Marwan Sabbagh, MD
View ›Lee Jen Wei, PhD
View ›CONTACT US
CORPORATE ADDRESS
New York Office
1185 Avenue of the Americas, 3rd Floor
New York, NY 10036
(973) 242-0005
INVESTOR RELATIONS
Neil Cataldi
E-mail: ncataldi@synaptogen.com
















